A few days ago, under the leadership and guidance of the subject leader, Professor Xu Kailin, the Department of Hematology of the Affiliated Hospital of Xu Medical University, Dr. , Shanghai Jiaotong University, University of Texas Southwestern Medical Center, and Shanghai Longyao Biotechnology Co., Ltd., using multi-target and multi-type CAR-T cells to sequentially treat a patient with advanced malignant lymphoma. One month after the re-infusion of cells, the mass in the patient's body disappeared, and the relevant indicators returned to normal after re-examination, which marked the success of the treatment. It is reported that this is the first patient with advanced lymphoma in the world who achieved complete remission after sequential multi-target and multi-type CAR-T cell therapy.
The patient Zhou Hua (pseudonym), female, 62 years old, was diagnosed with diffuse large B-cell lymphoma (germinal center origin, stage IV) due to "multiple masses throughout the body" in April 2016, and her chromosomes showed complex karyotype. After repeated chemotherapy, the disease control was not satisfactory. PET-CT showed bilateral parotid glands, nasopharynx, oropharynx, bilateral submandibular, submental, inner edge of sternocleidomastoid muscle, bilateral posterior neck triangle, Supraclavicular area, right scapula subcutaneous, bilateral axilla, mediastinum, bilateral hilum, right heart diaphragm angle, bilateral posterior diaphragm angle, between liver and stomach, around pancreas, root of mesentery, around abdominal aorta, right There were multiple lymphadenopathy in the mesocolic area, bilateral iliac vessels, bilateral inguinal area, and perirectal mesangial area, and the glucose metabolism was significantly increased, suggesting that the tumor had extensive systemic invasion and the prognosis was very poor.
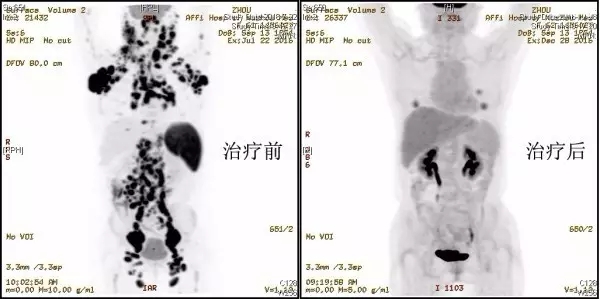
The patient's conventional treatment has no obvious effect, and the prognosis is very poor even after salvage autologous hematopoietic stem cell transplantation. The family members of the patients put their last hope in CAR-T cell therapy. In August 2016, the patient was infused with IL-7+IL-15 co-cultured CD19 CAR-T cells, and the tumor shrunk briefly and then swelled again. In September 2016, the infusion of CD19 CAR-T cells cultured with IL-2 was used, and the patient's mass remained unchanged. At this time, the patient and his family were in despair and were ready to give up the treatment. After repeated discussions, the medical team decided to try CD20 CAR-T therapy in combination with international cutting-edge theories. Half a month after the infusion of CAR-T cells in November 2016, the swollen lymph nodes in the patient's body have completely subsided, and the treatment process has mild side effects. At present, the cells have been reinfused for one month, and the re-examination PET-CT showed that many masses have disappeared, and the patient has achieved complete remission. Dr. Cao Jiang believes that the treatment experience of this patient suggests that sequential or combined multi-target CAR-T cell therapy will hopefully bring good news to more advanced lymphoma patients.
Shanghai Longyao Biotechnology Co., Ltd. is committed to the research and development and transformation of immune cell technology and biomedical technology. Carry out in vitro optimized culture and preclinical animal experiments of different target CART technologies, and jointly carry out multi-center clinical research on refractory B-cell hematological malignancies with a number of clinical institutions. In terms of experimental technology, the transfection efficiency of the CAR gene was optimized, the efficient expansion of CAR-T cells was achieved, and the level of memory T cells (Tcm) in the product cells was effectively increased, so that the CAR-T cells had better ability after infusion into the body. Long survival time and sustained killing effect; and after specific expansion, non-specific T cells are diluted to further reduce side effects.
The success of CAR-T in the treatment of lymphoma will further promote the research progress of cellular immunotherapy. Longyao Bio will also continue to work hard and assist clinical institutions to use this technology to benefit more tumor patients.