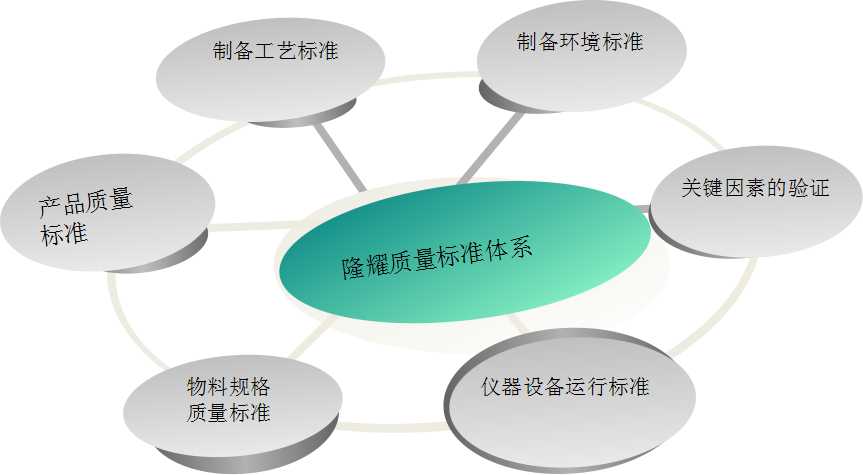
The Longyao biological team has many years of experience in quality control management in the field of pharmaceuticals and cell preparations, and has participated in and presided over a number of certifications, including the American AABB system, ISO9001, GMP certification, etc., to effectively monitor various key points and ensure products. quality.
Reference Standard
GMP
American AABB
State Food and Drug Administration formulated in 2003 "Technical Guidelines for Human Cell Therapy Research and Preparation Quality Control"
The third category of medical technology management regulations formulated by the Ministry of Health in 2009 "Autologous immune cells (T cells, NK cells) GMP U.S. AABB
Guideline for human cellular clinical research and reagents quality control, 2003, SFDA. Ministry of Health develop a third class medical technology management practices therapeutical techn-ical management standard of autologous immune cells (T cells, NK cells), 2009, Department of Health Guidance for Human Somatic Cell Therapy and Gene Therapy, 1998, US FDA