Chimeric Antigen Receptor (CAR)

He is a professor of the Department of Pathology at the University of Chicago, a researcher at the Institute of Biophysics, Chinese Academy of Sciences, and a special-appointed professor of the "Thousand Talents Program" of the Organization Department of the CPC Central Committee in 2009. In the past five years, he has presided over 8 NIH large-scale funded projects, with a total research funding of more than 10 million US dollars. He has published a large number of important and innovative research papers, and has become one of the few doctors and famous scholars at the University of Chicago who have made outstanding achievements in both clinical and scientific research fields. More than 140 SCI articles have been published, and the papers have been cited as many as 3700 times. Especially in the past five years, he has published more than 30 papers in top academic journals with SCI factors above 15 such as Natrue Science, Nature Medicine, Natrue Immunology, and mmuni1y.
technical background
CAR is a recombinant antigen receptor composed of an antigen binding domain and a T cell receptor signaling domain. The specificity and function of T lymphocytes can be re-regulated by CAR gene modification. Once expressed on T cells, the CAR will confer on T cells to recognize allogeneic antibodies in an MHC-independent manner
At the same time, it also bypasses the mechanism by which many tumor cells escape immune surveillance, so as to recognize and kill tumor cells most efficiently.
CAR technology is currently recognized as the most effective targeted immune cell therapy technology. Clinical studies on hematological malignancies such as ALL and CLL have shown a complete remission rate of about 90%.
The third-generation CD20-CAR technology jointly developed by Shanghai Longyao and Professor Fu Yangxin of the University of Chicago will conduct clinical application research on B-cell lymphoma, ALL and CLL.

1. CAR structure - the third generation of CAR technology
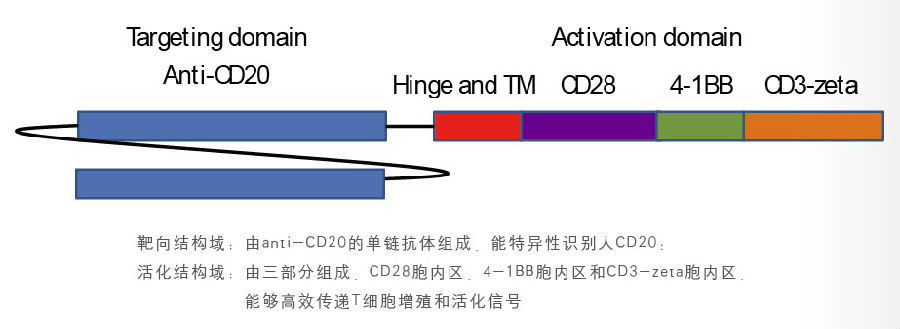
2. Production and optimized storage of CD20-CAR lentivirus
At present, the virus titer of about 1*108 infectious units/ml can be obtained, and the virus activity is greater than 97% within two months.
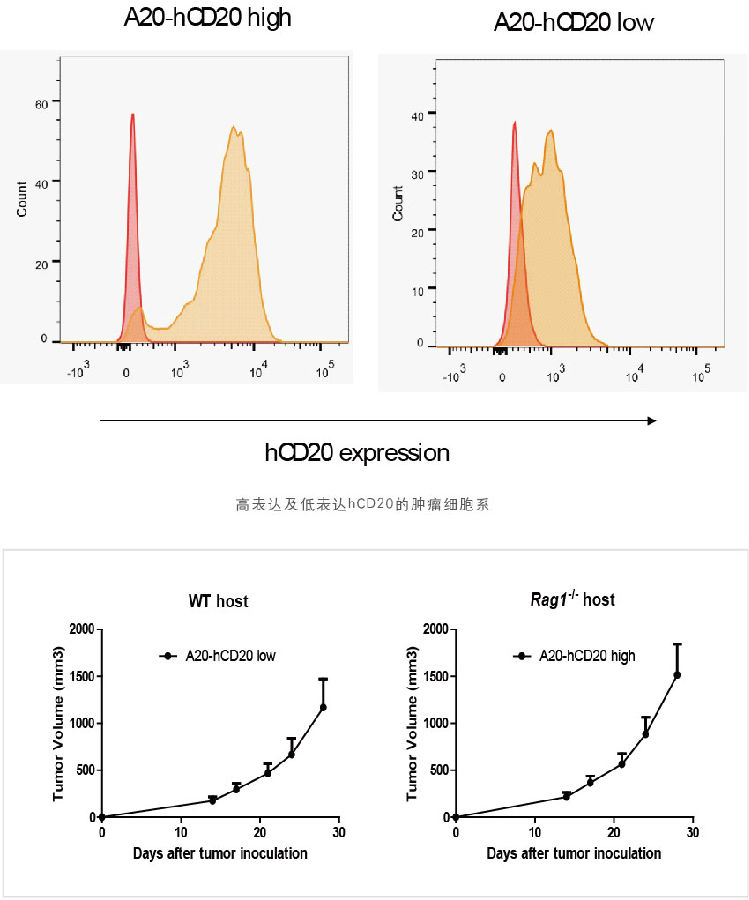
3. Established a mouse animal model of B-cell lymphoma that can express both high and low hCD20
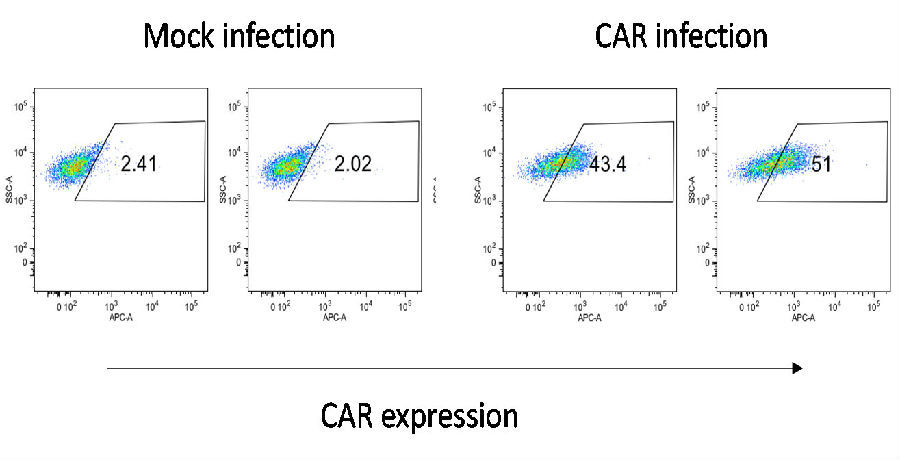
4. An optimized virus transfection process has been established, and the transfection efficiency reaches 20-50%.
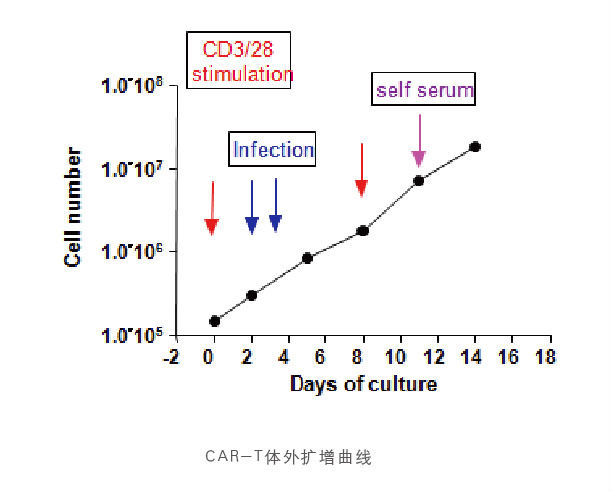
5. An optimized CAR-T in vitro amplification process has been established, which can be amplified by about 1000 times within 2W
The patented (Invention Patent No.:ZL 2018 1 0636409.0、ZL201980041270.8、US 11.590.168 B2)chemical technology will be used to effectively increase the level of memory T cells (Tcm) in vitro expansion, so that CAR-T cells will have longer survival time and sustained killing effect after infusion into the body.
CAR-T Tips
CAR-T (Chimeric Antigen Receptor-T cells)
Chimeric Antigen Receptor T Cell Technology
The surface of T cells carries antibodies that recognize tumor-specific antigens, enabling T cells to directly recognize and bind tumor cells, without the need for MHC, to target and kill tumor cells.
Mainly treat tumors:
B-cell lymphoma, acute lymphocytic leukemia ALL, chronic lymphocytic leukemia CLL, melanoma, etc. CD19/CD20-CAR
CAR-T advantages:
Targeting: The process of identifying and killing tumor cells is not restricted by MHC;
High energy: It has efficient killing effect, one CAR-T cell can kill more than 1000 tumor cells;
Long-acting: CAR-T can survive for a long time in the body for several months, and can expand more than 1000 times in the body, which has the effect of continuously killing tumors
Clinical treatment effect
In 2011, June et al first reported:
Using second-generation CD19-CAR-modified T cells to treat 3 patients with CLL, 2 patients achieved complete remission and 1 patient had stable disease. CAR-modified T cells expand more than 1,000-fold in vivo and maintain activity for 6 months, and some CAR-modified T cells even exist in the form of memory cells.
The New England Journal of Medicine 2014.10.16
Novartis-funded CD19-CAR research:
A total of 30 ALL patients were enrolled, and 27 patients achieved complete remission, with a complete remission rate of 90%. The 6-month event-free survival rate was 67%, and the overall survival rate was 78%.